
Pharmaceutical packaging companies play a critical role in the pharmaceutical industry. They are largely responsible for keeping pharmaceutical products safe and secure as they travel through the supply chain. Thus, choosing a packaging company to...
Read more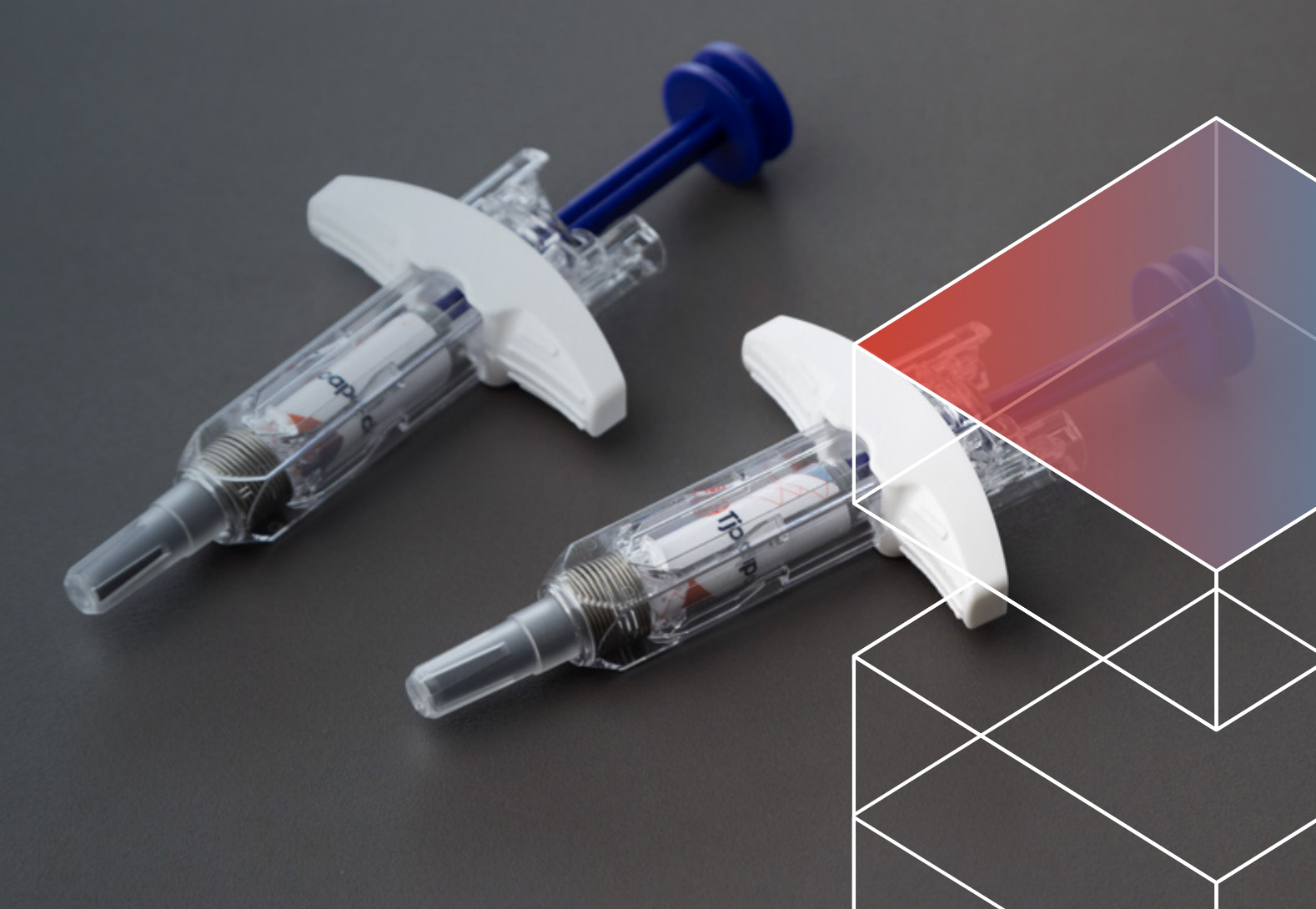
Download our latest whitepaper

The customer approached Tjoapack to develop a packaging solution for launching their product in the US market. They requested an evaluation of their current packaging and the design of a new solution that complies with the applicable...

The project entailed assisting the client with packaging small volumes of product to facilitate their expansion into the Chinese market. The first challenge was preparing the product to meet the specific requirements of the Chinese market, which...

We received a request to develop secondary packaging for vials containing a controlled substance used to manage moderate to severe cancer pain across multiple markets. The client specified the need for packaging that ensures the integrity of a 50R...

A request for primary and secondary packaging of capsules of a drug used for the prevention and treatment of secondary hyperparathyroidism. The project was at the early development stage and the client asked for the full set of validations with...

The challenge of the project revolves around a significant mismatch in the size of the packaging components. Specifically, the leaflet was too large, while the vial was too small. This caused a problem because, despite the size discrepancy, the...

Pharmaceutical packaging companies play a critical role in the pharmaceutical industry. They are largely responsible for keeping pharmaceutical products safe and secure as they travel through the supply chain. Thus, choosing a packaging company to...

It’s extremely essential to protect pharmaceutical products from damage during shipping and distribution, as faulty medication could have disastrous consequences for consumers. That’s why the drug packaging process is so important, and must be carrie

Pharmaceutical packaging plays a critical role in the medical supply chain. It protects pharmaceutical products from physical damage and chemical alterations throughout the shipping and distribution process, keeping them safe and effective for...

The pharmaceutical industry requires some unique packaging services that companies like Tjoapack are equipped to provide. One of these services is pharmaceutical kit assembly.Because pharmaceutical kitting involves assembling all of the elements...

If you have ever taken any kind of medication, be it prescription or over-the-counter (and you almost certainly have), then you’re probably familiar with blister packaging. Blister packaging is extremely common in the pharmaceutical industry...

Tjoapack and technology start-up company, Veratrak, have launched the European Platform on Changing Healthcare (EPOCH) in Europe. The initiative is designed to further connect the pharmaceutical industry, encourage cross partner collaboration and...

Postponement packaging is the practice of keeping products in a semi-finished, market agnostic state, and only finalising them when there is a specific market demand.Used strategically, it will allow pharmaceutical manufacturers to respond to...

Postponement packaging, or late-stage-customisation, is the supply chain practice of keeping a product in agnostic packaging until the last moment, only making it market specific when demand for the product arises. This prevents over or under...

Up until recently, outsourced drug manufacturing and packaging of products would be the responsibility of contract manufacturing organisations (CMOs). However, this is beginning to change, as contract packaging organisations (CPOs) are taking a...

Serialization, or track and trace legislation, has been introduced to the pharmaceutical industry due to global concerns over patient safety and the number of counterfeit drugs currently in the market. However, these are not the only benefits to...

The World Health Organisation (WHO) estimates that up to one in ten medicines could be counterfeit in poorer countries. On top of patient safety concerns, this is costing $30bn a year. To combat this issue, the EU will be introducing the Falsified...

Postponement packaging, or late-stage customisation, is a system that allows drug firms to respond to fluctuating market demand as quickly as possible, as products are kept in a standard format until the moment a demand for them emerges. This...

As the European Falsified Medicines Directive (EU FMD) compliance deadline gets closer, companies who have not yet prepared for serialization will have no choice but to outsource to meet the requirements in time. Tackling serialization...

The Drug Supply Chain Security Act (DSCSA) will be actively enforced in just six months, and it is set to revolutionise how drugs are distributed throughout the US. Companies that are yet to develop an in-house solution are realizing that...

One of the most prevalent issues in the industry today, many pharma businesses are talking about serialization. However, a recent survey has found that 42% of contract manufacturing organisations (CMOs) are not prepared for the changes that the...

With 6 months to go until the DSCSA serialization enforcement deadline kicks in, time is short for pharma companies and contract manufacturers that have not yet developed an in-house solution. Manufacturing Chemist spoke to Dexter Tjoa, Director...

As the deadlines for the US and European serialization regulations near, pressure is mounting on businesses throughout the pharma supply chain to finalize their solutions. It’s also the case that many businesses are looking beyond compliance and...

The deadlines for the US Drug Supply Chain Security Act (DSCSA) and EU Falsified Medicine Directive (FMD) are fast approaching. These regulations will further the integrity of the global drug supply chain, however, many pharma companies, contract...

Many pharma businesses understand the significance of serialization and have taken the necessary steps to prepare for the important legislation. However, a recent survey has found that not one of the EU Falsified Medicines Directive (FMD) pharma...

Widely adopted by the automotive industry, postponement is a logical and beneficial step forward for CPOs, particularly those who are running products in lower volumes or with an unpredictable demand. At first, the process may appear cumbersome...

2017 has been a year full of change for the pharmaceutical industry. One of the biggest challenges has been the implementation of serialization ahead of the European Falsified Medicines Directive (EU FMD) and the US Drug Supply Chain and Security...

Over the last decade the demand for contract packaging services has grown substantially with the market expected to exceed $100 billion by 2019. With more and more complex molecules entering the drug pipeline, the need for experienced packaging...

Serialization has been widely discussed by the pharmaceutical industry over the last five years and as the regulatory deadlines in Europe and the US get closer, the challenges associated with developing and implementing a robust solution are...

Serialization deadlines are getting closer and the complex task of implementation has been gravely under-estimated by many in the industry. There is a common misconception that implementing serialization is as simple as adding a barcode to the box...

There’s been a huge amount of discussion surrounding including three-tier aggregation as part of serialization solutions, even for markets where it is not a legislative requirement. Our CEO, Eric Tjoa, believes that despite aggregation adding...

Despite the FDA announcement that it will delay the active enforcement date of the DSCSA by a year, companies who have yet to start implementing a solution are still facing an uphill battle to compliance. In this industry roundtable with...

Serialization legislation has been introduced in a bid to eradicate counterfeit medicines from the pharmaceutical supply chain. The counterfeit medicines market is estimated to be worth around $200 billion annually, demonstrating that the cost and...

The announcement of the FDA’s decision to delay the active enforcement date for the Drug Supply Chain Security Act (DSCSA) is not a huge shock. There has been widespread discussion surrounding how prepared the industry is to implement the...

February 9 2018 marked one-year until the EU Falsified Medicines Directive (FMD) enforcement date, meaning the timeframes are tight for companies who haven’t already started implementation. As a result, many small and mid-sized pharmaceutical...

The pharmaceutical packaging landscape has seen a lot of changes in recent years. The advent of new technologies has allowed companies to explore new solutions and improve their operational efficiency. In this article with Contract Pharma, Dexter...
Ready to shape the future of your pharmaceutical supply chain?